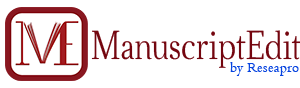Clinical Chemistry 2018
About Conference
EuroSciCon welcomes contributions on the progress in fundamental and applied research and cutting-edge clinical laboratory medicine as it invites all the participants from all over the world to attend "3rd Edition of International Conference on Clinical Chemistry and Molecular Diagnostics" during April 16-17, 2018 at Amsterdam, Netherlands which includes prompt keynote presentations, Oral talks(Speaker Forum and Young research Forum), Poster presentations, Symposium, Workshops and Exhibitions.
Clinical Chemistry 2018 is a global overview the Theme: "Recent developments and applications in Clinical Chemistry and Molecular Diagnostics" is focused on novel teaching and training methods applicable to laboratory medicine and is designed for professionals at all levels and career phases of the Clinical Chemistry research areas, who want to improve their understanding of what will drive and shape the future of the market. It will bring together researchers and practitioners from academia, industry, informal science, and governmental and non-governmental agencies to address practical and theoretical issues related to the teaching and learning of chemistry relevant to a variety of educational contexts and levels. As is expected for the very highly regarded EuroSciCon Conferences, presented work is intended to be current and unpublished, representing the cutting-edge of research and practice in this field. The conference format includes invited plenary sessions, semi-structured poster sessions, and free time to interact and network with conferences. Several features of the conference create an atmosphere that is intended to invigorate professional connections and promote advancement of the field: daily forums with intense intellectual engagement, off-the-record discussion of unpublished work, free afternoons in an idyllic setting, common meals and dormitory accommodations, and a limit on participation
Target Audience for Clinical Chemistry 2018:
Eminent Scientists/ Research Professors in the field of Clinical Chemistry, Junior/Senior research fellows, Students, Directors of Clinical Research Areas, Clinical Chemistries, Members of Clinical Chemistry associations and exhibitors from Clinical Research Industry/ Clinical Research Industries.
Why to attend our Conference:
Routine clinical chemistry and Molecular Diagnostics comprises 30 to 40 tests of substrates, metabolites, electrolytes, blood gases, enzymes and lipids that can be organized into panels for specialty patient investigation and routine patient screening. Common specialty panels include those for renal clearance, liver function and metabolic status. Clinical chemistries are performed on analyzers with varying levels of automation depending on the test volume run at a particular test site.
The market for routine clinical chemistries can be segmented in high and low volume users. High volume users are found in large urban and regional hospital and private laboratories. Low volume users are found in non-laboratory tests sites within the hospital, physician office labs, small and medium sized hospitals and community laboratories.
The Market for Clinical Chemistry analyzes the current and potential market for clinical chemistry, featuring a market forecast for 2019. The report includes discussion of:
Overview of Chemistry Tests
Size and Growth of the Market
Market Trends
Leading Products and Prices
Innovations
Physician Office Labs (POLs)
Laboratory Automation
This mature industry is continuously trying to reinvent itself by improving test processes and test menus. Market growth is modest in spite of predicted explosive growth in emerging markets because developed nations account for a very large share of the market. Leading competitors and product developers are listed in the manufacturer profiles. The profiled companies include
Abbott Laboratories
Beckman Coulter/Danaher
Hitachi High-Technologies Corp
Instrumentation Laboratory
Ortho-Clinical Diagnostics
Roche Diagnostics
Siemens Healthcare
Sessions/Tracks
Track 1: Clinical Biochemistry
Clinical Biochemistry is a special branch of medicine dealing with measurement and interpretation of the physicochemical condition and dynamics in healthy and diseased humans, thus contributing to a pathophysiological understanding and thereby to prophylaxis, diagnosis, therapy, prognostication and research of disease. Many diseases show significant changes in the chemical composition of body fluids such as the raised blood enzymes due to their release from heart muscles after a heart attack; or a raised blood sugar in diabetes mellitus due to lack of insulin. Biochemical tests are designed to detect these changes qualitatively or quantitatively compared to results from healthy people. Clinical biochemistry use a broad range of analytical techniques for example, molecular diagnostics, measurement of enzyme activities, spectrophotometry, electrophoresis, the separation of molecules based on physical characteristics and immunoassays. A journal is a periodical publication intended to further progress of science, usually by reporting new research. Most journals are highly specialized, although some of the oldest journals publish articles, reviews, editorials, short communications, letters, and scientific papers across a wide range of scientific fields.
Track 2: Clinical Genomics
Clinical Genomics provides an overview of the various next-generation sequencing (NGS) technologies that are currently used in Clinical and Molecular Diagnostics laboratories. It presents key bioinformatic challenges and the solutions that must be addressed by clinical genomicists and genomic pathologists, such as specific pipelines for identification of the full range of variants that are clinically important. Clinical genomics is the use of genome sequencing to inform patient molecular diagnosis and care. Clinical genomics is a new and rapidly-changing field. Knowledge of the human genome is far from complete, but there are already uses for genetic and genomic information in the clinic. Genome sequencing is expected to have the most impact in characterising and molecular diagnosing rare and inherited disease stratifying individuals’ tumours to guide treatment (precision medicine) providing information about an individual’s risk of developing disease or their likely response to treatment (health management). A major focus of genomic medicine is cancer diagnosis and therapy. Clinicians are beginning to use genomic information to predict how a person's cancer will respond to drug therapy or surgery. In some cases, clinicians will profile the DNA and RNA of tumour cells to guide the use of existing treatments or focus on more targeted treatments.

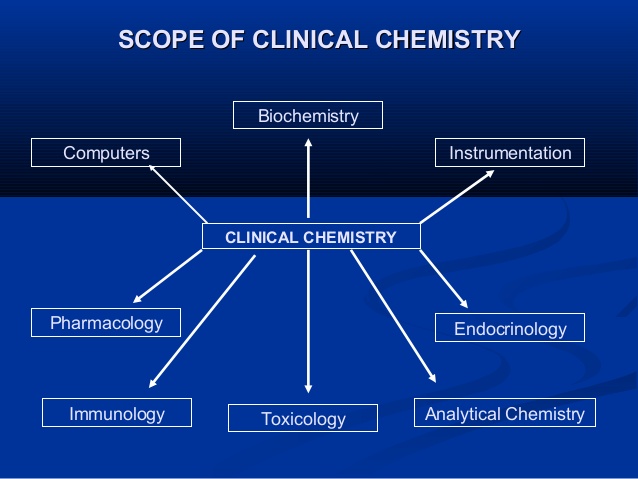
Track 3: Clinical Immunology and Autoimmunity
The clinical practice of Immunology, as defined by the World Health Organisation (WHO) encompasses clinical and laboratory activity dealing with the study, diagnosis and management of patients with diseases resulting from disordered immunological mechanisms, and conditions in which immunological manipulations form an important part of therapy. The clinical work of Immunologists is largely out-patient based and involves primary immunodeficiency, allergy, autoimmune rheumatic disease and systemic vasculitis (jointly with Rheumatologists), joint paediatric clinics for children with immunodeficiency and allergy and immunoglobulin infusion clinics for patients with antibody deficiency. On the laboratory front, Consultant Immunologists are responsible for directing diagnostic immunology services and perform a wide range of duties including clinical liaison, interpretation and validation of results, quality assurance and assay development.

Clinical immunology is the branch of Immunology that deals with studies of diseases caused due to immune system disorders. Clinical Immunology falls into two categories Immunodeficiency and Autoimmunity. Immunodeficiency is a category in which adequate response is not provided by the immune system. Whereas in Autoimmunity the immune system attacks its own host body.
Autoimmunity occurs when an organism develops an immune response against itself, resulting in an inflammatory reaction which damages organs such as brain, joints or pancreas. This results in diseases such as Type 1 diabetes, vasculitis, or rheumatoid arthritis. A fine balance exists in order to accommodate the control of microbial pathogens and commensals, and immune self-tolerance.
Track 4: Clinical Microbiology
Clinical Microbiology is a branch of medical science concerned with the prevention, diagnosis and treatment of infectious diseases. Moreover, this field of science is concerned about various clinical applications of microbes for the improvement of health. There are four kinds of microorganisms that cause infectious disease: bacteria, fungi, parasites and viruses. It is the adaptation of microbiological techniques to the study of the etiological agents of infectious disease. In this one can explore nature of infectious disease and test the ability of various antibiotics to inhibit or kill the isolated microorganisms. The invasion and multiplication of microorganisms such as bacteria, viruses, and parasites that are not normally present within the body is called infection. Now days due to mutation and continuous adaptability of microbes to the changing environment there are vast number of diseases that are to be studied for betterment of the humankind. Research in clinical microbiology mainly includes studying various aspects of microbes including virulence factor of the microbe.

Track 5: Therapeutic Drug Monitoring and analysis
Therapeutic Drug Monitoring and analysis is a measurement of drug concentrations as a guide to the optimal prescription of drugs. Ensuring that patients are taking the most effective dose of a drug is key to any drug treatment plan. Assays have been developed to measure the active pharmaceutical ingredient, and, where appropriate, metabolites that have a pharmaceutical effect or reflect key parameters of excretion. We offer validated assays supported by participation in external proficiency testing programmes for a variety of different drugs, including the following groups:- Antifungals, Antibiotics, Cardiac Drugs Immunosuppressant. In addition to the healthcare sector, we also offer a prompt service for patients undergoing rehabilitation from drugs of abuse. Our therapeutic drug monitoring assays have been developed to ensure that results are delivered within the clinically relevant time period.

Track 6: Evaluation of Molecular Diagnostic Biomarkers
A biomarker is a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. It used to detect or confirm presence of a disease or condition of interest or to identify individuals with a subtype of the disease. Medical practice requires accurate diagnosis of diseases and conditions. Molecular Diagnostic Biomarkers are used for the critical determination of whether a patient has a particular medical condition for which treatment may be indicated or whether an individual should be enrolled in a clinical trial studying a particular disease. As is becoming increasingly appreciated, many diseases have subtypes with markedly different prognoses or responses to a specific treatment. Purpose or uses of a test or biomarker:
1. Diagnosis,
2. Disease classification,
3. Risk stratification,
4. Disease prognosis,
5. Treatment stratification,
6. Treatment monitoring and
7. Population screening.
Benefits: A new body should be established to ensure the evaluation of molecular diagnostic tests
A publically available database be created of new and existing laboratory tests – a ‘molecular diagnostics formulary’ – containing evidence for clinical performance, and explicitly stating where any evidence is lacking. Policy makers and industry should be encouraged to address issues around gathering the necessary evidence for clinical evaluation.

Track 7: Disease-oriented Topics (Cardiovascular Disease, Cancer Diagnostics, Diabetes)
Collectively, cardiovascular disease (including stroke), cancer, and diabetes account for approximately two-thirds of all deaths in the United States and about 700 billion US dollars in direct and indirect economic costs each year. Current approaches to health promotion and prevention of cardiovascular disease, cancer, and diabetes do not approach the potential of the existing state of knowledge. A concerted effort to increase application of public health and clinical interventions of known efficacy to reduce prevalence of tobacco use, poor diet, and insufficient physical activity-the major risk factors for these diseases-and to increase utilization of screening tests for their early detection could substantially reduce the human and economic cost of these diseases. In this article, the American Cancer Society, the American Diabetes Association, and the American Heart Association review strategies for the prevention and early detection of cancer, cardiovascular disease, and diabetes, as the beginning of a new collaboration among the three organizations. The goal of this joint venture is to stimulate substantial improvements in primary prevention and early detection through collaboration between key organizations, greater public awareness about healthy lifestyles, legislative action that results in more funding for and access to primary prevention programs and research, and reconsideration of the concept of the periodic medical checkup as an effective platform for prevention, early detection, and treatment.

Track 8: New Reagents, Instrumentation & Technologies
Clinical chemistry reagents and instruments that utilize the latest formulations and technologies for quantitative determination of substrates, enzymes, and electrolytes in human serum, plasma, or urine. To complement our general chemistry reagents, it has both semi-automated analyzers and fully-automated analyzers. On the basis of products and services, the market is segmented into reagents & kits, instruments, and services & software. The reagents & kits market is expected to grow at the highest CAGR of the market in the forecast period. Growth in the reagents & kits segment is driven by advancements, such as automation and high-throughput technologies in instruments.
Based on technology the market is segmented into PCR, INAAT, microarrays, hybridization, DNA sequencing, Next-generation sequencing (NGS) and other technologies (electrophoresis, flow cytometry, and mass spectrometry). Among these technologies, PCR is expected to command the largest share and microarray is expected to be the highest growing segment. The large share of this segment is primarily attributed to the growing use of PCR in proteomics & genomics, automation of PCR instruments, and emergence of advanced technologies like qRT-PCR.

Current and Emerging Products
Analysis of current and emerging clinical chemistry and molecular diagnostic tests. Review of current instrumentation technologies, and feature comparison of high-, medium-, and low-volume/POC analyzers.Technology Review: Assessment of current and emerging technologies, and their potential market applications.Companies developing or marketing new technologies and products by test.

Track 9: Clinical Pathology
Clinical Pathology cares with the identification, treatment, and interference of health problem. Clinical pathologists unit doctors with special coaching job World Health Organization sometimes direct all of the special divisions of the laboratory research, which might embrace the bank, clinical chemistry and biology, toxicology, haematology, medical specialty and medical science, and biological science. Hematopathology or hemopathology is that the branch of pathology that studies diseases of hemopoietic cells. Transfusion is that the branch of medication that is committed the transfusion of blood and blood components. Medical biological science may be a branch of medication committed the hindrance, identification and treatment of infectious diseases. Immunopathology may be a branch of medication that deals with immune responses reated with unwellness. It includes the study of the pathology of associate degree organism, organ system, or unwellness with regard to the system, immunity, and immune responses.

Track 10: Clinical Research
Clinical Research is a part of healthcare science that determines the safety and effectiveness (efficacy) of medications, devices, diagnostic products and treatment regimens intended for human use. These may be used for prevention, treatment, diagnosis or for relieving symptoms of a disease. Clinical research is different from clinical practice. In clinical practice established treatments are used, while in clinical research evidence is collected to establish a treatment. Clinical research refers to the entire catalogue of a drug/device/biologic, in fact any test article from its inception in the lab to its introduction to the consumer market and beyond. Once the promising candidate or the molecule is identified in the lab, it is subjected to pre-clinical studies or animal studies where different aspects of the test article are studied. Different types of clinical research are used depending on what the researchers are studying. Below are descriptions of some different kinds of clinical research.

Treatment Research generally involves an intervention such as medication, psychotherapy, new devices, or new approaches to surgery or radiation therapy.
Prevention Research looks for better ways to prevent disorders from developing or returning. Different kinds of prevention research may study medicines, vitamins, vaccines, minerals, or lifestyle changes.
Diagnostic Research refers to the practice of looking for better ways to identify a particular disorder or condition.
Screening Research aims to find the best ways to detect certain disorders or health conditions.
Quality of Life Research explores ways to improve comfort and the quality of life for individuals with a chronic illness.
Epidemiological studies seek to identify the patterns, causes, and control of disorders in groups of people.
Track 11: Clinical Pharmacology and Toxicology
Clinical pharmacology deals with study of drugs and their clinical use. It is supported by the basic science of pharmacology, with added focus on the application of pharmacological principles and quantitative methods. It has a wide scope, from the discovery of new target molecules to the effects of drug usage in whole populations. Clinical Toxicology is processes with are involved with the different forms of toxic chemicals and they associated with the different forms of diseases. It typically coincides with other sciences like as biochemistry, pharmacology and pathology.

Clinical Pharmacology and Toxicology aims to develop coherent means to modify drug therapy, with respect to the patients genotype, and to ensure maximum efficiency with minimal contrary effects. Clinical Pharmacology & Toxicology is the all-encompassing and becoming an increasingly important discipline for the identification of disease targets and drug designing with their toxicological effects and means to eradicate diseases. It would address the issues and provide the readers with the essentials of the science and theory behind these disciplines, also giving practical applications of various methods for better understanding of modern drug design and development. It concentrates on the complicated aspects, psychological and most probably physical addiction to drugs despite of its detrimental consequences and increased tolerance with their toxicological effects and their therapies.

Track 12: Clinical and Hospital Pharmacy
Clinical pharmacy is a health science discipline in which pharmacists provide patient care that optimizes medication therapy and promotes health, and disease prevention. The pharmacist verifies the legality, safety and appropriateness of the prescription order, checks the patient medication record before dispensing the prescription (when such records are kept in the pharmacy), ensures that the quantities of medication are dispensed accurately, and decides whether the medication should be handed to the patient, with appropriate counselling, by a pharmacist. The pharmacist can participate in arrangements for monitoring the utilization of drugs, such as practice research projects, and schemes to analyze prescriptions for the monitoring of adverse drug reactions. Hospital pharmacies are pharmacies usually found within the premises of a hospital. Hospital pharmacies usually stock a larger range of medications, including more specialized and investigational medications (medicines that are being studied, but have not yet been approved), than would be feasible in the community setting. Hospital pharmacies typically provide medications for the hospitalized patients only, and are not retail establishments. The practice of pharmacy that responds to the needs of the people who use the pharmacists’ services to provide optimal, evidence-based care. To support this practice it is essential that there be an established national framework of quality standards and guidelines.

Track 13: Market of Clinical Chemistry
The major changes in clinical chemistry have been brought about as a result of the convergence of system engineering, automation, and IT technology. Thus, new technologies have enabled a better understanding of disease processes. The clinical chemistry market is expanding, consolidating, and becoming highly competitive with a myriad of opportunities for various new instruments, reagents, calibrators, and other systems.
Clinical chemistry and molecular diagnostic markets are undergoing significant transformation, caused by convergence of new and more stringent regulations; advances in diagnostic technologies, system engineering, automation, and IT; and intensifying competition. Some segments, like routine chemistry, are already resembling commodity markets, where product positioning and cost per test are more critical than underlying technology. This evolving marketplace creates exciting opportunities for a variety of new instruments, reagent systems, and auxiliary products, such as specimen preparation devices, controls, and calibrators.

Market Analysis
The geographic segments included in this report are Americas, Europe, BRIC, Japan and Rest of the World (RoW).
Rise in number of lifestyle diseases, automation of laboratories will aid the growth of this market. The market is expected to be driven by increasing awareness for preventative care, increase in aging population, increase in reagent rental agreements and laboratory automation.
The “Clinical Chemistry and Molecular Diagnostics Analyzer Market by Product (Systems & Reagents), Test Type (Basic Metabolic, Electrolyte, Liver, Lipid, Renal, Thyroid Function, Specialty Chemicals) & by End User (Hospitals, Laboratories, Academic Research) - Global Forecast to 2019” report provides a detailed overview of the major drivers, restraints, challenges, opportunities, current market trends, and strategies impacting the global clinical chemistry analyzers market along with the estimates and forecasts of the revenue and market share analysis.
Clinical Chemistry Analyzers Market worth $11,728.01 Million by 2019.
The global clinical chemistry and Molecular Diagnostics market is growing due to the ongoing developments in analytical laboratory automation, the swift progress in various fields of diagnosis such as point-of-care testing, molecular diagnosis, immunoassays, hematology, flow cytometry, and microbiology, and finally, the geographical market expansion within emerging countries.
The most important trend witnessed recently in the in vitro diagnostics (IVD) industry is the trend of self-testing as opposed to patients visiting hospitals. This is one of the biggest factors responsible for the growth of point-of-care testing, as patients prefer self-testing so as to avoid unnecessary visits to the hospital. Clinical chemistry accounted for 21.3% share of the IVD market in 2012. Clinical chemistry routine tests such as testing for blood albumin, ALT/SGPT, ammonia, blood gases, and calcium and creatinine levels, are required before undergoing advanced tests. These tests, thus, form the center stage of the IVD market.
The major changes in clinical chemistry have been brought about as a result of the convergence of system engineering, automation, and IT technology. Thus, new technologies have enabled a better understanding of disease processes. The clinical chemistry market is expanding, consolidating, and becoming highly competitive with a myriad of opportunities for various new instruments, reagents, calibrators, and other systems.
The Center for Medicare & Medicaid Services (CMS) has estimated that healthcare spending in the U.S. is expected to grow from $2.7 trillion in 2011 to about $4.6 trillion in 2019, at a CAGR of 6.8% for the same period. Similarly, in emerging countries, the growing awareness and an increasing middle-class population, with a more disposable income to spend on healthcare, will be the major driving factors for the growth of this market.
The report on the global clinical chemistry and Molecular Diagnosticsmarket analyzes the market by three segments, namely, clinical chemistry reagents, instruments, and accessories. All three segments experienced a positive growth till 2013, with a market value of $10.3 billion, comprising $8.74 billion for reagents and $1.63 billion for instruments. The overall market is projected to grow at a CAGR of 8.3% during the forecast period.
The Americas commanded the largest share (42%) of the global clinical chemistry market at an estimated $4,332.3 million in 2013, expected to reach $6,050.6 million by 2018, at a CAGR of 7.2% from 2013 to 2018. In Europe, Germany commanded the largest share (20%) at an estimated $652.8 million in 2013, expected to reach $853.3 million by 2018, at a CAGR of 5.8% from 2013 to 2018. The APAC clinical chemistry market is growing in double digits and will continue to grow in the future. China commanded the largest share (53%) at an estimated $516.81 million in 2013 and is expected to reach $1,047.2 million by 2018, at a CAGR of 16% from 2013 to 2018.
The global molecular diagnostics market is projected to reach USD 10.12 Billion by 2021 from USD 6.54 Billion in 2016, at a CAGR of 9.1% from 2016 to 2021. In this report, the market is broadly segmented on the basis of technology, applications, products & services, end user, and region.
The report also provides an extensive competitive landscape of the leading companies operating in this market. The main companies extensively covered in this report are Abbott Laboratories, Alere, Inc., ARKRAY, Inc., Bayer AG, Becton, Dickinson and Company, bioMérieux, Bio-Rad Laboratories, Inc., and Danaher Corporation, among others. The details of segment and country-specific company shares, news and deals, mergers and acquisitions, segment-specific pipeline products, product approvals, and product recalls of the major companies have been covered in the report.
Report Customization Options
Along with market data, you can also customize MMM offerings that are in keeping with your company’s specific needs. Customize your report on the global clinical chemistry market for to get an insight into all-inclusive industry standards and a deep-dive analysis of the following considerations:
Opportunity Analysis:
Unmet needs, revenue pockets, and potential areas for expansion
Supplier Evaluation:
Comprehensive review of key suppliers
Lab-Testing Data:
Number of clinical chemistry tests performed annually in each country, tracked till sub-segment level
Current and Emerging Products:
An analysis of current and emerging clinical chemistry and immunodiagnostic tests
A review of current clinical chemistry instrumentation technologies and feature comparison of high, medium, and low-volume POC analyzers
Product Analysis
Usage pattern (in-depth trend analysis) of products (segment wise)
Product matrix which gives a detailed comparison of the product portfolio of each company mapped at country and sub-segment level
End-user adoption rate analysis of the products (segment wise and country wise)
Comprehensive coverage of product approvals, pipeline products, and product recalls
Brand/Product Perception Matrix
Comprehensive study of customers perception and behavior through our inbuilt social connection tool checking the virality and tonality of blogs
Analysis of overall brand usage and familiarity and brand advocacy distribution (detractor/neutral/familiar)
Alternative Products: Impact Analysis
MMM’s Healthcare Decision Making Quadrant: It is an innovative and useful quadrant for vendors who wish to analyze potential growth markets based on parameters like patient dynamics (patient pool, epidemiology of disease, preference towards surgeries/alternative therapies) and macroeconomic indicators (number of hospitals and orthopedic clinics, reimbursement scenario, diagnosis rate, treatment rate, and healthcare expenditure).
Learn More
Top Clinical Chemistry Universities Worldwide:
Clinical Chemistry Universities:
Harvard University | University of Cambridge | University of Oxford | Massachusetts Institute of Technology | Stanford University | Johns Hopkins University | Karolinska Institute | University of California | University of California | Yale University | University College London | Imperial College London | The University of Melbourne | University of California Berkeley | University of Toronto | University of California San-Diego | The University of Sydney | King’s College London | Duke University | National University of Singapore | University of Edinburgh | The University of Tokyo | University of Pennsylvania | University of Washington | Cornell University | Columbia University | University of Copenhagen | McGill University | Monash University | University of British Columbia | University of Michigan | The University of Queensland | Washington University in St Louis | University of Hong Kong | Seoul National University | University of Amsterdam | University of Chicago | London School of Hygiene & Tropical Medicine | University of North Carolina | Kyoto University |
Euroscicon Ltd with immense pleasure invites all the contributors across the globe to the 2nd International conference on Food Security and Sustainability (Food Security 2017) during June 26-27, 2017 at San Diego, USA which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions.
Euroscicon Ltd organizes 1000+ scientific events inclusive of 600+ Conferences, 500+ Workshops and 200+ Symposiums on various topics of Science & Technology across the globe with support from 1000 more scientific societies and Publishes 500+ Open Access journals which contains over 50000 eminent personalities, reputed scientists as editorial board members. Food security is often defined in terms of food availability, food access and food utilization. Global agriculture currently produces ample calories and nutrients to provide the entire world's people healthy and productive lives". However, food is not distributed equally to regions, countries, households and individuals. Improved access to food-through increased agricultural productivity and incomes-is essential to meet the food needs of the world's growing population. Successful food security and poverty-oriented programmes not only assist poor rural populations to produce more and diversified products but to produce a surplus that can be marketed and thereby generate income for the purposes of improving quality of life through improved diet and nutrition, investment in productive activity, and as collateral for credit to purchase inputs and/or other supplies to enhance agricultural or non-agricultural enterprise. Agricultural economists have maintained that greater concentration on small farmers leads to faster growth rates of both aggregate economic output and employment .Other analysts argue that production-focused service delivery directed solely at the poor as producers in isolated areas will yield low and probably diminishing returns. San Diego is a major city in California, on the coast of the Pacific Ocean in Southern California. San Diego is the eighth-largest city in the United States and second-largest in California With an estimated population of 1,394,928 as of July 1, 2015, San Diego is the birthplace of California and is known for its mild year-round climate, natural deep-water harbour, extensive beaches, long association with the United States Navy and recent emergence as a healthcare and biotechnology development center. The city is the seat of San Diego County and is the economic center of the region. Join us at Global Food Security conference for “Producing sustainable thoughts to bolster the future”. This event has been designed to address scientists, scholars, and different societies supporting food security, Industries and other related scientific communities with different levels of awareness, expertise and proactive solutions to create global impact in this field. Moreover, it will help industrialists to incorporate sustainability into every aspect of Agricultural Industries business model. The Food Security conference will influence industries to maximize their yield and profit through the application of strategic techniques. Additionally, it will reveal the best techniques to promote sustainable agricultural development and achieve a hunger free world by 2050.
We look forward to an exciting scientific event in the beautiful city of San Diego, USA.Market analysis